DIAGNOSE 3 -
TRÄNEN-FILM DIAGNOSTIK
=> hier finden Sie eine vereinfachte Übersicht Diagnostischer Verfahren für das Trockene Auge
Tränen-Film Stabilität
Break-Up Time - BUT / Tränenfilm Aufbruchszeit - TAZ
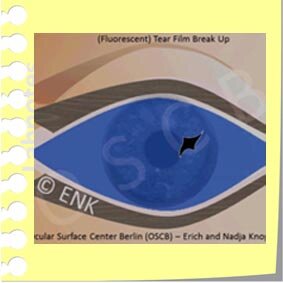
Die Tränenfilm Aufbruchszeit (BUT) ist einer der wichtigsten funktionellen Werte für die Intaktheit und Gesundheit der Augenoberfläche. Sie beschreibt die Zeit in Sekunden in der der Tränenfilm intakt nach einem kompletten Lidschlag bis zu seinem ersten Aufbruch an irgendeiner Stelle.
Sobald der Tränenfilm aufbricht, beginnen die empfindlichen Zellen der Augenoberfläche sofort zu trocknen, Schaden zu nehmen und entsprechend den Reiz über die Nervenfasern an das Gehirn zu senden. Dies löst dann entsprechende Beschwerden des Patienten im Sinne eines Gefühls von Irritation oder sogar Schmerz aus.
Wie in den Kapiteln über die ´Augenoberfläche´ sowie´Tränen und Tränenfilm´ beschrieben wird, ist ein intakter Tränenfilm eine Grundvoraussetzung für die Gesundheit der Augenoberfläche und auch für eine perfekte Sehschärfe.
FLUO-BUT (F-BUT)
When the Tear Film BREAK-UP Time (BUT) is estimated conventionally, the vital stain FLUORESCEIN is used to make the tear film visible in order to see its Break-Up - this is termed Fluorescent BUT (F-BUT). A Break-Up typically occurs in a small area and quickly becomes larger. In the region of the Break-Up the epithelial cells are exposed to the ambient air and are prone to dry out. The exposure of the epithelium is immediately sensed by the sensory nerves and initiates an eye blink to remove the old ruptured tear film and to spread a new one.
Üblicherweise wird die Stabilität des Tränenfilms geprüft durch die Gabe des Vitalfarbstoffs Fluoreszein für den sogenannten FLUO-BUT Test. Der Fluoreszenzfarbstoff dient dazu, die Oberfläche des Tränenfilms ausreichend sichtbar zu machen an der Spaltlampe, bei Beleuchtung mit blauem Licht.
Fluoreszein wird typischerweise als kleines Tröpfchen aus einer Flasche gegeben, so dass dieser Test einfach und einigermassen wiederholbar und zuverlässig ist.
Abgesehen davon. dass der Tränenfilm durch die Zugabe von des Stoffes Fluoreszein bereits verändert wird, ist dieser Test nur dann einigermassen zuverlässig, wenn möglichst einige Bedingungen konstant gehalten werden:
die Menge von Flüssigkeit, die in den Tränenfilm gegeben wird
die Konzentration von Fluorescein
Effektiv kann die Fluoreszein-Konzentration auf dem Auge leicht variieren, abhängig von der Tränen-Menge auf der Augenoberfläche.
Ein kleiner Nachteil ist, dass einige Patienten nicht unbedingt begeistert sind von der Anwendung von Fluoreszein, da dies ein sehr intensiver Farbstoff ist. Er kann die Lidränder noch für eine Weile anfärben kann ... oder es gibt die Befürchtung, dass er das Make-up ruinieren mag ... aber was ist das schon im Vergleich zur grossen Nützlichkeit dieses Tests !
Da die Zugabe eines Fluoreszein-Tropfens den Tränenfilm bereits verändert wird faktisch nicht die ´wahre´ Aufbruchszeit gemessen, sondern eine ´künstliche Aufbruchszeit´. Wenn der Test aber standardisiert, immer hinreichend gleich, durchgeführt wird, sind die Ergebnisse recht zuverlässig. Es verbleibt nur ein kleiner systematischer Fehler. Daher hat der FBUT Test in den letzten Jahrzehnten viele wertvolle Ergebnisse zur Augenoberfläche und dem Trockenen Auge erbracht.
Tränenfilm-Stabilität: Normalwert: > 10 Sekunden (überlicherweise, wissenschaftlich wird teils auch 5 Sekunden als Grenze verwendet)
TOPOGRAPHERS are Multi-Purpose devices that are stand-alone, are typically equipped with different illumination systems for different purposes and are connected to a computer with an appropriate software system for evaluation and storage of examination results. Certainly very useful for Dry Eye Diagnostics is the possibility to perform Non -Invasive Tear Film Break-Up (NI-BUT) measurements that allow the measurement of Tear Film stability without interfering with it by addition of marker any substances.
BUT - Nicht-Invasiv (NI-BUT)
Die Nicht-Invasive BUT (NI-BUT) bietet alle Vorteile, die bei der herkömmlichen Fluo-BUT fehlen.
Es erfordert jedoch eine spezielle Ausrüstung, die es erlaubt, die Tränenfilm-Lipidschicht sichtbar zu machen auch ohne Anfärbung.
Dazu wird typischerweise ein Muster (meist Placido-Ringe) auf die Oberfläche des Tränenfilms projiziert und mit einer Video-gesteuerten Computeranalyse die Veränderung der Reflexion ausgewertet.
Vorteile der NI-BUT
Da der Tränenfilm vor der Untersuchung nicht verändert wird, liefert die NI-BUT prinzipiell einen Wert für die Tränenfilm Stabilität, der ´näher an der Wahrheit´ liegt.
Typischerweise bleibt der Tränenfilm etwas länger stabil ohne Fluoreszienzugabe. Faktisch sind die Werte aber meist nicht wesentlich unterschiedlich.
Es werden keine zusätzlichen Reagentien benötigt
Das zur Analyse verwendete Gerät macht typischerweise eine automatische Messung und liefert einen numerischen Wert. Allerdings sind auch automatische Messungen nicht unbedingt fehlerfrei.
Nachteile der NI-BUT
Es wird eine spezielle apparatetechnische Ausrüstung benötigt zur Darstellung und Analyse des Tränenfilms. Je nach Anspruch und Leistung ist dies mehr oder weniger kostspielig.
Die erforderliche Ausrüstung ist typischerweise ein sogenannter Topographer (siehe Abbildung)
Der Topographer ist ein Multifunktionsgerät, das auch noch anderer Aufgaben wahrnehmen kann, wie OF-Topographie zur Kontaktlinsenanpassung, und Bestimmung anderer Parameter für die Diagnose des Trockenen Auges. Diese vielseitigen Geräte sind ohnehin für einen modernen Augenoberflächen- und Sicca-Spezialisten nahezu unverzichtbar.
Tränenfilm Lipidschicht Untersuchung
Die Tränenfilm-Lipidschicht (TFLL) ist die oberflächliche Phase des dreischichtigen Tränenfilms
The view of pro-ocular Tear FILM as a basically three layered liquid film is the leading concept for roughly the last 70 years since Eugene WOLFFS original description in 1946 ... and this concept still appears to hold true.
Die oberflächliche Schicht oder Phase des Tränenfilms besteht aus Lipiden.
Sie haben, nach heutigem Kenntnisstand, hauptsächlich die Funktion die Verdunstung der darunter liegenden wässrigen Hauptphase der Tränen zu verzögern. Lipide können auch dazu beitragen, die z.B. die Ausbreitung der Tränen zu einem dünnen Film zu unterstützen.
Die wässrige Phase aus den Haupt- und akzessorischen Tränendrüsen ist zum darunter liegenden Epithel hin zunehmend mit sezernierten gelbildenden Schleimstoffen (Muzin) aus den Becherzellen vermischt.
Insgesamt führt dies zu dem heutigen Konzept des Tränenfilms als einer mehr oder weniger dreischichtigen Struktur. Dies geht im Wesentlichen auf Eugene WOLFF zurück, der dieses Konzept 1946 entwickelte ... in einer Zeit, in der sich wahrscheinlich nicht allzu viele Menschen zu sehr an solchen Fragen interessiert waren.
Erst der sehr dünne gleichmässige Tränenfilm erlaubt die Herstellung einer glatten Oberfläche für eine perfekte Lichtbrechung auf der Oberfläche des Auges. Dies ist die Basis für eine perfekte Sehschärfe.
Die Tränenfilm Lipidschicht besteht aus 2 Sub-Schichten mit unterschiedliche Eigenschaften
The superficial layer of the tear film is composed of lipids with possibly some minor amounts of associated proteins. The lipid film is very thin but is still conceptually separated into a much thicker outer layer of non-polar lipids that appear to represent the ´lid on the pot of warm tears´ that retards aqueous evaporation. Underneath is a much thinner sub-layer of non-polar lipids, possibly with minor contents of proteins, that can connect the above layer to the underlying aqueous phase.
Die Lipidschicht ist sehr dünn ... im Bereich von etwa 100 Nanometern (nm) - das ist nur etwa die 10-fache Dicke einer Zellmembran. Dennoch scheint sie aus zwei Teilschichten zusammengesetzt zu sein.
Die äußere dicke Schicht besteht aus unpolaren hydrophoben Lipiden
Die äußere dickere Unterschicht ist aus unpolaren und damit hydrophoben Lipiden zusammengesetzt. Die äußere dickere Unterschicht scheint aus unpolaren und damit hydrophoben Lipiden zusammengesetzt zu sein, was zu dem Problem führt, dass diese Lipide sich nicht an das darunter liegende Wasser binden können.
Die innere Schicht hat polare Lipide, um die Verbindung mit der wässrigen Unterphase aufrechtzuerhalten
Daher ist eine innere Verbindungsschicht aus Lipiden notwendig, die sich an ihre Lipidpartner an der Oberfläche, aber auch an das darunter liegende Wasser binden kann. Es gibt auch einige Proteine, die bei dieser Funktion der Bindung hydrophober Lipide an eine darunter liegende Wasserphase helfen können.
Die Dicke der Ölschicht auf dem Tränenfilm kann gemessen werden durch die Analyse der Interferenzfarben (Interferometrie)
Die schematische Darstellung einer normalen Tränenfilm-Lipidschicht in Interferometrie zeigt ein wirbelndes Muster von rötlich-goldenen Interferenzfarben. Die Interferenz ist typischerweise am besten zu sehen, wenn das Raumlicht gedimmt ist. Weiter unten finden Sie ein echtes Foto einer Interferometrie.
Die Analyse der Lipid-Menge auf dem Tränenfilm ist möglich durch die Messung der Dicke dieses Lipidfilms.
Die Dicke der Lipidschicht wiederum lässt sich durch die Messung der Interferenzfarben bestimmen. Dies sind ´Falschfarben´, ähnlich den schillernden Farben eines Ölflecks auf einer Wasserlache oder den Farben an der Oberfläche einer Seifenblase. Die Farben stehen in direkter Beziehung zur Dicke der Ölschicht. Sie ist sehr dünn - im Bereich um etwa 100 Nanometer, also ein Zehntausendstel Millimeter.
Die Messung der Dicke der Lipidschicht ist daher glücklicherweise einfacher als die Bestimmung der Dicke des gesamten Tränenfilms, über die in der Vergangenheit sehr unterschiedliche Werte ermittelt wurden und die bis heute nicht ganz klar ist.
Unter geeigneter Beleuchtung produzieren Schichten im Dickenbereich um 100 Nanometer Interferenzfarben die von grau (sehr dünn) über silber und blau bis zu gelb und rot (sehr dick) reichen - siehe Abbildung.
Beim Lidschlag wird die Lipidschicht zusammengeschoben und bei der Lidöffnung entfaltetet sie sich wieder.
INTERFEROMETRIE in der klinischen Praxis - der Gold-Standards der Lipid-Schicht Analyse des Tränenfilms
The Analysis of the Tear Film LIPID LAYER (TFLL) by INTERFEROMETRY is already available since the 1980s by a simple to use handheld device - the TearScope, that was invented by the Tear Film Specialist Jean-Pierre GUILLON. Apart from estimating the thickness of the Lipid Layer it is also possible to obtain the Non-Invasive Tear Film BREAK-UP Time (NI-BUT).
Theoretisch ist es möglich, die Interferenzfarben der TFLL mit einer gewöhnlichen Spaltlampe sichtbar zu machen, wenn die Beleuchtung manuell angepasst wird, um genügend Streulicht zu erzeugen ... aber dieses Verfahren ist typischerweise nicht sehr genau und nicht sehr befriedigend.
Zwei hervorragende Interferometer wurden von klinischen Spezialisten entwickelt
TearScope
Das erste praktisch einsetzbare Gerät scheint das TearScope (hergestellt von Keeler Inc.) zu sein. Dabei handelt es sich um ein Handgerät, das Mitte der 1980er Jahre von Jean-Pierre GUILLON entwickelt und 1984 auf dem legendären Tear Film & Dry Eye Congress in Lubbock, Texas, vorgestellt wurde.
Mit dem Tear Scope lassen sich der Tränenfilm und seine Lipidschicht sehr detailliert untersuchen. Einige Jahre später wurde eine verbesserte Version, das TearScope 'Plus', entwickelt.
Das allgemeine Interesse an der Lipidschicht des Tränenfilms war jedoch während dieser Zeit und später begrenzt, so dass weltweit zu wenige Instrumente verkauft wurden und die Produktion später eingestellt wurde.
Mit dem zunehmenden Interesse an der Meibom Drüsen Dysfunktion (MDD) und den Tränenfilm Lipiden wurde ein neues 'Advanced TearScope' verfügbar.
Dieses ist auf dem neuesten Stand der Technik und erlaubt somit die Verbindung zu einem mobilen Tablet-Computer, um die gewonnenen Daten aufzuzeichnen und zu dokumentieren. Dies ist sicherlich von Vorteil für eine nahtlose Integration in den klinischen Arbeitsablauf.
LipiView
Das interferometrische Bild der Tränenfilm-Lipidschicht, das hier mit dem TearScience Lipiview erstellt wurde, zeigt, dass ein Tränenfilm mit normaler Dicke eine Mischung von Interferenzfarben aufweist, die hauptsächlich rötlich-golden sind (oberes Bild). Dies entspricht einer durchschnittlichen Dicke von 90 Nanometern. Die Lipidschicht im unteren Bild ist zu schmal, wie an der grauen Interferenzfarbe" zu erkennen ist - die automatische Messung zeigt eine durchschnittliche Dicke von 35 Nanometern.
Ein weiteres Interferometer wurde etwa 20 Jahre später ebenfalls von einem engagierten klinischen Spezialisten für die Tränenfilm-Lipidschicht entwickelt - dies war Donald KORB.
Korb und Kollegen entwickelten das Instrument in der klinischen Praxis entsprechend den praktischen Bedürfnissen des Klinikers.
Ziel war es, ein Instrument zu entwickeln, das höchste Standards in der Bildqualität aufweist, um höchste Messgenauigkeit zu erreichen. In Verbindung mit bestem Komfort in der Anwendung und Dokumentation der Ergebnisse, um sich nahtlos in den praktischen klinischen Ablauf einzufügen. Dieses Gerät wurde später von der Firma TearScience unter dem Namen Lipiview produziert.
Darüber hinaus kann dieses Gerät auch die eine Darstellung der Meibomdrüsen im Augenlid des Patienten (Meibographie) mit makelloser Perfektion durchführen.
Die Lipiview erfasst automatisch die Lipidschicht des Tränenfilms über ein weites Sichtfeld in einem Film. Dabei wird auch der Lidschlag der Augen, einschließlich partieller Lidschläge aufgezeichnet. Partielle, inkomplette Lidschläge (“Nervöses Blinzeln”) sind ohne Hilfsmittel nur schwer sicher zu beobachten, stellen aber vermutlich einen unterschätzten Risikofaktor für ein Trockenes Auge dar.
Das Lipidview führt eine vollautomatische Analyse der Daten durch, die in einem Bericht ausgedruckt werden kann (siehe Abbildung).
Die Analyse der Tränenfilm-Lipidschicht ist ein wichtiger Bestandteil einer aussagekräftigen Untersuchung des trockenen Auges
Die Entwicklung des Lipiview-Gerätes fiel in die Zeit, als man schließlich erkannte, dass ein Mangel an den Tränenfilm-Lipiden bei der überwiegenden Mehrheit der Patienten die Hauptursache für die Erkrankung des trockenen Auges ist. Dies bezieht sich auf etwa 4 von 5 Patienten mit trockenem Auge, die an dieser Hauptursache leiden, und unterstreicht, dass die Analyse der Tränenfilm-Lipidschicht eine wichtige und notwendige Komponente einer aussagekräftigen Untersuchung der Augenoberfläche darstellt. Die Hauptursache für Lipidmangel, wie sie im TFOS MGD-Bericht (frei verfügbar unter: www.tearfilm.org) bewertet wird, ist die obstruktive Dysfunktion der Meibomdrüsen, die als Meibomdrüsen-Dysfunktion bezeichnet und meist mit MGD abgekürzt wird).
Seit dem TFOS-MGD-Bericht im Jahr 2011 ist das Interesse an der Tränenfilm-Lipidschicht (Tear Film Lipid Layer, TFLL) zu Recht fast explodiert, nachdem erkannt worden war, dass die Störung der Lipid-produzierenden Meibom-Öldrüsen (Meibomdrüsen Dysfunktion, MDD) in den Augenlidern, die Hauptursache für ein Trockenes Auge ist.
Dies hat zur Entwicklung mehrerer neuer und praktischer Geräte für die Tränenfilm- und TFLL-Analyse geführt. Diese Entwicklung hat die wichtige Diagnostik des Tränenfilms und der Tränenfilm Lipidschicht für jeden Kliniker, der sich mit der Augenoberfläche befasst, zugänglich gemacht.
Tear Osmolarity Evaluation
Öl Mangel führt typischerweise zu erhöhter Tränen-Verdunstung mit Hyperosmolarität
Zustände an der Augenoberfläche mit einem Mangel an Tränenfilm Lipiden haben eine ausgedünnte Ölschicht auf dem Tränenfilm, die dann zu einem Verlust der Verdunstungshemmung führt. Das Tränenwasser verdunstet dann schneller (blaue Pfeile) und die gelösten Stoffe (Salze und Proteine - in der Abb. als weisse Kugeln) werden stärker konzentriert. In der Folge (1) reduziert sich die Menge der Tränen auf dem Auge und der Tränen-Meniskus sinkt, was typischerweise zu verringerter ´Schmierung´ zwischen Augenlid und Augapfel beim Lidschlag führt, und damit zu einer erhöhten mechanischen Reibung. Zusätzlich (2) führt die Konzentrierung der Salze in den Tränen zu einer erhöhten Osmolarität, auch als ´Hyperosmolarität´ bezeichnet. Diese übt einen Wasser-entziehenden osmotischen Effekt auf die empfindlichen Zellen aus, der sie schädigt. Sowohl erhöhte Reibung wie auch erhöhte Osmolarität stellen einen Entzündungsreiz dar. und können zum Fortschreiten des Trockenen Auges zu einer chronischen Entzündungskrankheit beitragen.
Bei einem Ölmangel in der obersten Tränenfilmschicht kommt es zu erhöhter Verdunstung (Hyperevaporation) der wässrigen Hauptphase des Tränenfilms.
Dies führt zu einer zunehmenden Salzkonzentration/ Osmolarität (Hyperosmolarität) der verbleibenden Tränenflüssigkeit (siehe Abbildung).
Der Grund dafür ist, dass nur das Tränenwasser verdampft, ähnlich wie der Wasserdampf aus einem warmen Suppentopf. Dadurch wird der Rest der Tränenflüssigkeit immer konzentrierter und damit, wie im Fall der Suppe, zunehmend salziger.
Die Tränen enthalten neben verschiedenen Salzen zusätzlich andere Moleküle wie z.B. alle möglichen funktionellen und regulativen Proteine. Die Tränenproteine sind hydrophil und haben daher ebenfalls eine "wasseranziehende" kolloid-osmotische, konzentrierende Wirkung.
Diese Grundlagen wurden erstmals in den 1970er Jahren von Jeff GILBARD für den Tränenfilm erkannt, als wichtiger Pathomechanismus beim Trockenen Auge.
Das Konzept der Hperosmolarität wurde experimentell getestet und als Schädigungsmechanismus nachgewiesen. Therapeutisch wurde daraufhin vorgeschlagen, eine hypo-osmolare Tränenersatzmittel-Therapie anzuwenden. Diese relativ enthält mehr Wasser und weniger Salze als die normalen Tränen, und kann erfolgreich zur effektiven Therapie von hyper-evaporativen Erkrankungen des trockenen Auges angewendet werden.
Wie man heute weiss, stellen Lipidmangelstörungen mit erhöhter Verdunstung die Hautursache des Trockenen Auges dar (- siehe Fachpublikation dazu).
Hyper-osmolare Tränen sind ein Entzündungsreiz für das Gewebe der Augenoberfläche
In der Pathogenese des Trockenen Auges, ist die Hyperosmolarität (rechts in der Abb.) ein wichtiger Sekundärer Pathogenetischer Faktor. Hyperosmolarität ist die Folge der Primären Pathologie der Tränenfilmstörung mit Lipidmangel, die zu erhöhter Verdunstung des Tränenwassers führt.
Hyperosmolare Tränen üben eine wasserziehende Kraft auf die darunter liegenden Zellen aus. Der Wasserentzug setzt die Zellen unter einen hyperosmolaren Stress und aktiviert sie zu Abwehrmassnahmen.
Die Zellen reagieren auf die einzige Art und Weise, die sie gelernt haben zu reagieren - sie richten als grundlegende Schutzantwort eine Entzündung ein, wie im Kapitel über Entzündung erläutert.
Die gebildeten Botenstoffe der Entzündung (z.B. Zytokine) können sich in der Mikroumgebung der Augenoberfläche anreichern. Dies ist der Fall, wenn die schädliche Reizung chronisch wird, wie es beim Trockenen Auge typisch ist. So wird eine Entzündungsreaktion ausgelöst, wie von Steven PFLUGFELDERs Gruppe gezeigt wurde.
Das Entzündungsmilieu führt zum Übergang des Trockenen Auges in eine chronische immun-modulierte Schleimhautentzündung
Die schematische Abbildung aus einem Artikel von Mitgliedern der OSCB aus dem Jahr 2005 in "The Ocular Surface" erklärt die wichtigsten Schritte in der Pathogenese der immun-modulierten chronischen mukosalen Entzündung beim Trockenen Auge. Durch eine Schädigung des Epithels der Augenoberfläche werden Entzündungsmediatoren gebildet, die eine Reaktion des Schutzgewebes in Gang setzen, um den pathogenen Reiz zu beseitigen. Von hier aus wird eine Abfolge von sekundären Ereignissen ausgelöst, die (1) eine Anhebung von Antigen-Präsentationsmolekülen und (2) von Zelladhäsionsmolekülen, die den Einstrom von Zellen aus dem Gefäßkompartiment in das Gewebe induzieren und dort einen zerstörerischen Weg durch das Gewebe bahnen, sowie (3) die Aktivierung von bindegewebsabbauenden Enzymen (Matrix-Metalloproteinasen, hauptsächlich MMP9) im vermeintlich besten Interesse, den entzündungsschützenden Zellen Platz zu schaffen, umfassen.
All dies wäre kein großes Problem ... wenn die Veränderung nur akut und nicht chronisch fortschreitend wäre - was aber unglücklicherweise der typische Fall des Trockenen Auges ist. Daher ist die typische Entwicklung hier, dass sich die beabsichtigte Schutzreaktion in eine chronische immunologische Schleimhautentzündung umwandeln kann. Diese wird durch Lymphzellen, als normalem Schutzbestandteil des Gewebes, immunmoduliert wird und damit sehr stark und therapieresistent gegen konventionelle Sicca-Therapie mit ausschließlich Tränenersatzmitteln etc.. Dies ist die Grundlage für die sinnvolle Anwendung einer anti-entzündlichen Therapie beim chronischen Trockenen Auge.
Das Entzündungsmilieu im Gewebe führt zu einer Aktivierung des Bindegewebes unter der Deckschicht des Epithels. Dies betrifft Stromazelltypen und Gefäss-Endothelzellen mit Einwanderung weiterer Abwehrzellen in das Gewebe. Dies führt zu all den unglücklichen Ereignissen, die bei chronischen Schleimhautentzündungen und auch beim Trockenen Auge auftreten, wie sie von Mitgliedern der OSCB in ihrem Artikel TFOS Maui 2002 und im Artikel über die Augenoberfläche 2005 beschrieben wurden (siehe Fachartikel dazu).
Zu diesen Ereignissen gehört neben einer Stimulation von Lymphozyten des spezifischen Immunsystems auch die Produktion und Aktivierung von Protease-Enzymen wie MMP9, die die Gewebestruktur zerstören.
Tränenfilm Hyperosmolarität ist ein wichtiger Sekundärer Pathogenetischer Faktor für das Trockene Auge
Die schematische Abbildung zeigt die Pathophysiology beim Trockenen Auge, auf Basis des HOLISTISCHEN DYNAMISCHEN KONZEPTS. Grundlegende Kausale Faktoren stören die Bildung eines stabilen Tränenfilms durch Mangel an Tränenflüssigkeit oder durch gestörte Ausbreitung zu einem stabilen Film. Dies ist die typische Primäre Pathologie. Die Tränenfilmstörung führt zu sekundären schädlichen Faktoren wie Hyperosmolarität des Tränenfilms und erhöhter Reibung beim Lidschlag durch verminderte Schmierung. Schließlich führt dies zur zweiten Primären Pathologie der Beschädigung des Gewebes. Diese gilt ebenfalls als primär, da ein Gewebeschaden unter bestimmten Umständen auch zuerst auftreten kann und dann zu einer gestörten Anhaftung der Tränen an das Gewebe führt.
Auch wenn die Hyperosmolarität sicherlich ein wichtiger Faktor in der Pathophysiologie der Erkrankung des trockenen Auges ist, so ist sie doch kein "primärer" Faktor. Denn eine erhöhte Tränenosmolarität setzt immer etwas anderes voraus, das sie erzeugt, weil die Tränendrüse nicht primär hyperosmolare Tränen produziert.
Wie im Ganzheitlichen Dynamischen Konzept zur Hierarchie der Krankheitsfaktoren (siehe Abbildung) beim Trockenen Auges erläutert, ist die typische primäre Störung ein Tränenfilm-Mangel mit Mangel an Tränenfilm-Lipiden. Der Lipidmangel wiederum wird typischerweise durch eine Funktionsstörung der Meibomdrüsen (MDD) verursacht.
Tränenfilm Hyperosmolarität kann ein wichtiger Marker für ein Trockenes Auge sein
Tränenfilm Osmolarität ist ein wichtiger klinischer Parameter und wichtiger sekundärer pathogenetischer Faktorbeim Trockenen Auge. Die Messung kann heutzutage schnell und einfach mit einem hangehaltenen GErät (TearLab) durchgeführt werden. Es benutzt sterile Einmal Messspitzen zur Aufnahme der Tränenflüssigkeit.
Die Erkennung der Bedeutung eines Lipid-Mangels durch Verstopfung der Meibomdrüsen für die Erkrankung des trockenen Auges, erfolgte 1980 durch KORB und HERNANDEZ. Diese prägten auch die Bezeichnung "Meibomdrüsen Dysfuntion" (engl.: Meibomian Gland Dysfunktion, MGD).
Obowohl später Hyperosmolarität durch GILBBARD als wesentlicher pathologischer Faktor beim Trockenen Auge identifiziert wurde, war doch in den folgenden Jahren dieses Thema sozusagen "klinisch tot".
Erst gegen Ende des ersten Jahrzehnts nach dem Millennium wurde die Osmolarität wiederentdeckt. Bald galt sie als neuer "Goldstandard" für die Diagnostik der Trockenen-Augen-Krankheit.
Möglicherweise hing dies mit den früheren Problemen bei der Messung der Osmolarität zusammen, die nur mit großen und schweren Instrumenten in Laborumgebungen möglich war.
Dies hat sich mit der Erfindung eines kleinen, handlichen und einfach zu bedienenden automatisierten Instruments (TearLab) geändert. Dieses Gerät steht heute zur Verfügung, um die Tränenosmolarität sehr schnell und zuverlässig im klinischen Umfeld direkt am Patienten zu messen.
So wird die Osmolarität ein schnell und einfach zu bestimmender Parameter für die Diagnostik des Trockenen Auges und zur Verlaufskontrolle bei Therapie.
Das TearLab erlaubt die Messung der Tränenfilm Osmolarität schnell und direkt am Patienten
Die normale Tränenosmolarität liegt bei oder knapp unter 300mOsm/l (Milliosmol pro Liter) und kann schwanken um plus/minus 10mosm um 298 mOsm/l.
Zur Orientierung: Die Osmolarität anderer Körperflüssigkeiten ist z.B. mit 275-320mOsm/kg sehr ähnlich im Blutplasma aber, durch die notwendige Harnkonzentrierung, höher, d.h. > 400-500mos/l, im Urin.
Untersuchungen mit dem TearLab-Gerät haben gezeigt, dass ein Tränenosmolaritätswert über 306mOsm/l bereits als so erhöht gilt, dass er auf ein Trockenes Auges hinweist. Auch starke Unterschiede der Osmolarität zwischen beiden Augen gelten als verdächtig. Von Piera VERSURA und Kollegen sowie von vielen anderen Forschern wurde berichtet, dass ein zunehmender klinischer Schweregrad der Erkrankung des trockenen Auges mit steigenden Osmolaritätswerten korreliert. Die enge Korrelation der Tränenosmolarität mit dem klinischen Schweregrad der Augenerkrankung wird verständlich durch die oben beschriebene stark reizende Wirkung von hyperosmolarem Stress auf das Oberflächenepithel des Auges.